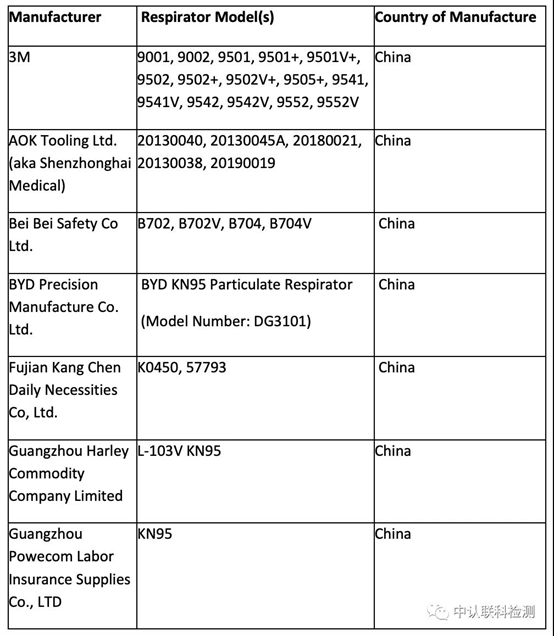
On May 7, the FDA again revised its China KN95 (FFR) mask recognition rules issued on April 3. And updated the products previously published in the white list (Appendix A), from the original 74 white list suddenly only 14 left! The reason is nothing more than that according to the results of the latest CDC (NIOSH) sampling test, there are a lot of problems with the filtration efficiency of KN95 masks from China. Let's take a look at those manufacturers who have survived the FDA+CDC double test and can still stay on the pound bill.
However, there is a major question at present. Can these 60 whitelisted factories not continue to export KN95 to the United States?
The FDA reissued the EUA today. In addition to greatly shortening the allowed import list, it also made the following amendments:
Amend the eligibility criteria to allow authorization based on acceptable performance of the standards of independent laboratory testing records;
Cancel the importer's ability to apply for EUA and instruct the manufacturer to provide a list of authorized importers;
Increase the recognition of the China National Drug Administration (NMPA) registration certificate, which is verified by the FDA.
The interpretation of these three new standards is:
If you have not successfully applied for the EUA, please use the NIOSH certificate, or the corresponding certification in other countries. The FDA can recognize the EN149 CE certificate, etc.
If you have been on the EUA but have now been canceled, you must get the NIOSH official breathing efficiency test (one of the standard NIOSH tests) or do a simplified version of the CDC version (and CDC random test) within 45 days Consistent), and the results are all above 95













