On December 14, 2021, the European Union issued Regulation (EU) 2021/2204 in its official gazette, which expanded the category 1B CMR (carcinogenic, mutagenic or reproductive toxicity) substances in items 28, 29 and 30 of Annex XVII of the REACH Regulation List.
Pursuant to the provisions of items 28, 29 and 30 of Annex XVII of the REACH Regulation, it is prohibited to put on the market and use 1A or 1B CMR substances listed in Annex 1 to Annex 6 of Annex VII, and it is prohibited to put on the market and use these substances and their concentration. Mixtures that exceed the specified limit. These substances are also listed in Part III of Annex VI of Regulation (EC) No 1272/2008.
The following substances have been added to Annexes 2, 4 and 6 of Annex XVII of the REACH Regulation as follows:
1. Attachment 2 (Item 28-Carcinogen: Category 1B)
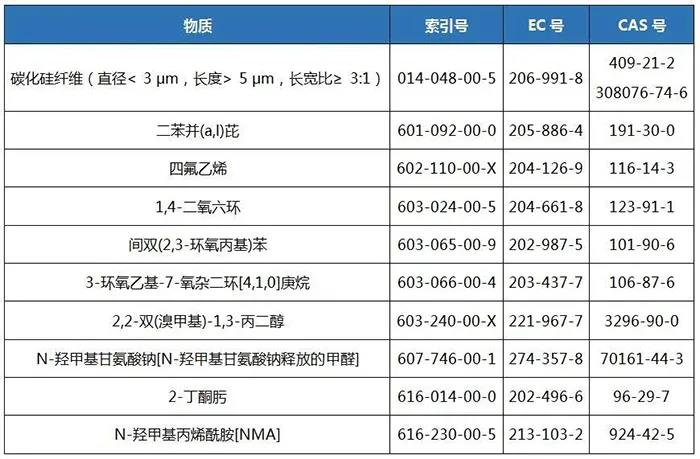
2. Attachment 4 (Item 29-Substances causing gene mutations in germ cells: Category 1B)
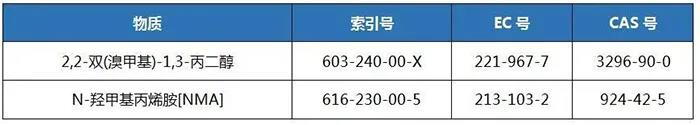
3. Annex 6 (Item 30-Reproductive Toxic Substances: Category 1B)
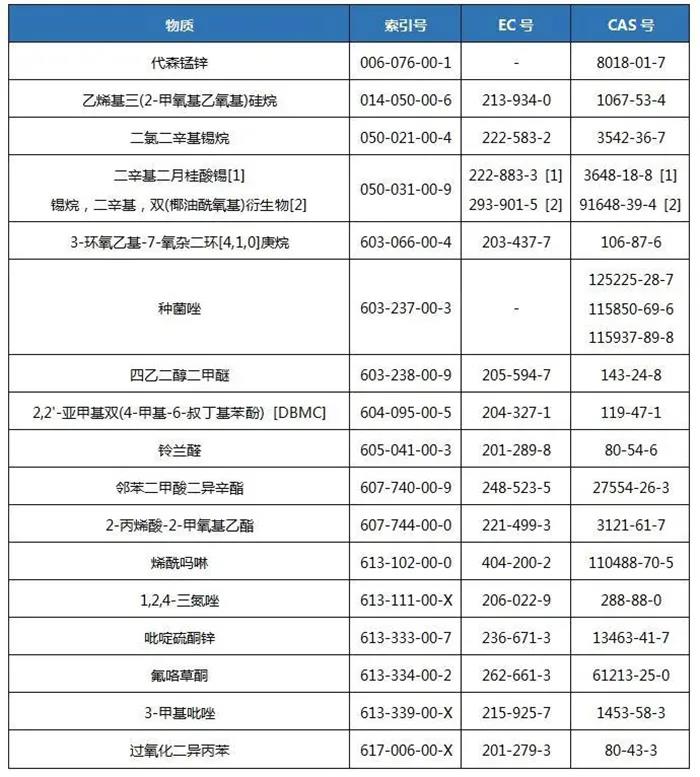
Reminder
With the continuous update of the CMR list in Annex XVII of the REACH Regulation, enterprises are facing more and more management and control requirements. ZRLK recommends that relevant companies improve their product risk awareness, pay attention to the update of the CMR list in Annex XVII of the REACH Regulation, and adjust production strategies. Ensure that the exported products containing restricted substances comply with the latest regulatory requirements and avoid trade risks!













