On March 4, 2022, the European Chemicals Agency (ECHA) announced a public comment on a potential Substance of Very High Concern (SVHC), and the comment period will end on April 19, 2022, during which various stakeholders Comments can be submitted to ECHA. Substances that pass the review will be included in the SVHC Candidate List as official substances.
The information on the substances under review is as follows:
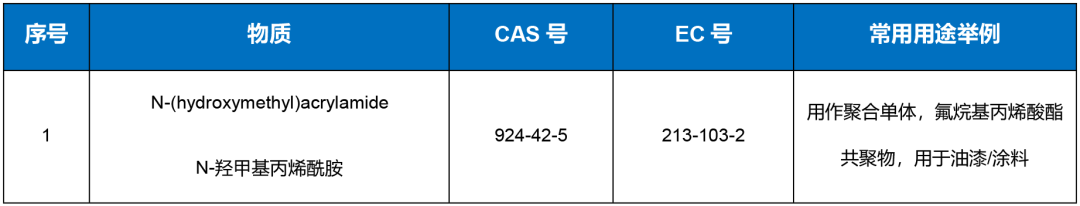
Reminder
The SVHC list is updated twice a year. With the continuous updating of the SVHC list, enterprises face more and more control requirements. Enterprises need to improve the risk awareness of their products. Within six months after the substances are listed on the SVHC list, qualified enterprises need to complete the SVHC notification in the articles. From January 5, 2021, article suppliers are required to submit to ECHA a SCIP notification of information on SVHC contained in articles. ZRLK recommends that companies investigate supply chains early to prepare for regulatory changes.













