On February 17, 2023, the European Chemicals Administration (ECHA) announced the public review of two SVHC candidate list of intended substances. The review period will end on April 3, 2023, during which all stakeholders can submit their comments to ECHA. The reviewed substances will be added to the official list of SVHC and become the 29th batch of SVHC substances.
The material information for this review is as follows:
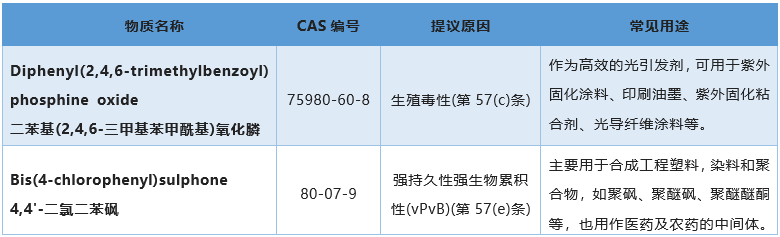
Relevant regulatory requirements for SVHC substances:
According to REACH regulations, if the content of SVHC in the product exceeds 0.1% (w/w), the information transmission obligation shall be fulfilled. If the content of SVHC in the article exceeds 0.1% (w/w), and the content of SVHC in the article exceeds 1 ton/year, the manufacturer or exporter shall report to ECHA.
According to the WFD requirements of the Waste Framework Directive: if the content of SVHC in the article exceeds 0.1% (w/w), the enterprise needs to make a SCIP notification from January 5, 2021, and the SCIP notification information will be published on the ECHA official website.
Warm tips
The SVHC list is updated twice a year. With the continuous updating of the SVHC list, enterprises face more and more control requirements. Enterprises need to improve their product risk awareness. Within six months after the substances are listed in the SVHC list, qualified enterprises need to complete the SVHC notification in the articles. From January 5, 2021, the article supplier shall submit to ECHA a SCIP notification of the relevant information of SVHC contained in the article. ZRLK recommends that enterprises investigate the supply chain as early as possible to cope with regulatory changes calmly.













