On October 2, 2023, ECHA announced a new SVHC intentional substance again, and planned to conduct public comment on the substance in February, 2024. The specific information of the substance is as follows:

Up to now, there are 15 published SVHC evaluation substances and intentional substances, including 7 evaluation substances and 8 intentional substances. It is worth noting that dibutyl phthalate (DBP) among the seven evaluated substances was added to SVHC as early as October 2008. This time, the second evaluation of this substance was initiated because it was identified that there was a new hazard type of this substance. So up to now, there are actually only 14 evaluation and intention substances that may be added to SVHC in the future, including 6 evaluation substances and 8 intention substances except DBP.
List of review substances
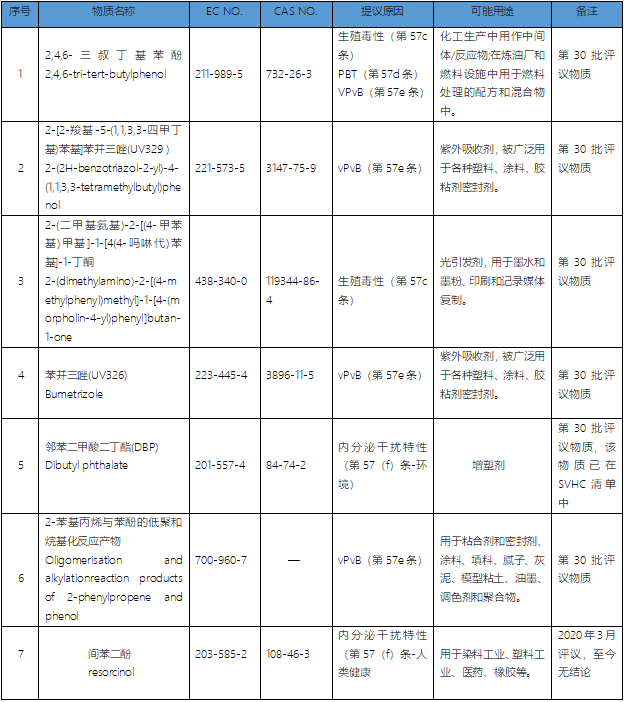
List of intentional substances

At present, these eight intentional substances are still in the stage of "the proposer prepares the dossier of related substances". Generally, ECHA will publish and start a 45-day public comment about one month after receiving the dossier of the proposal, and the reviewed substances after passing the final resolution of the European Commission will be added to the official list of candidate substances of SVHC.
Warm tips
With the continuous updating of SVHC candidate list, enterprises are facing more and more control requirements. ZRLK suggested that relevant enterprises should improve their product risk awareness, investigate the supply chain as early as possible, and always pay attention to the update of REACH regulations, so as to calmly cope with the changes in regulations. Our company has a professional technical team and rich experience in product testing, which can help you easily understand whether the products are safe and compliant. If you need it, please feel free to contact us, and our engineers will serve you at the first time!













