On December 4, 2023, the European Union issued the revised draft of Annex I of POPs Regulation (EU)2019/1021, and launched public feedback on the requirements of perfluorooctane sulfonic acid (PFOS) in Annex I of EU POP Regulation (EU) 2019/1021, mainly proposing to tighten the limit requirements of PFOS and cancel specific exemption clauses. The feedback period of the draft will end on January 1, 2024.
The main contents of this proposal are:
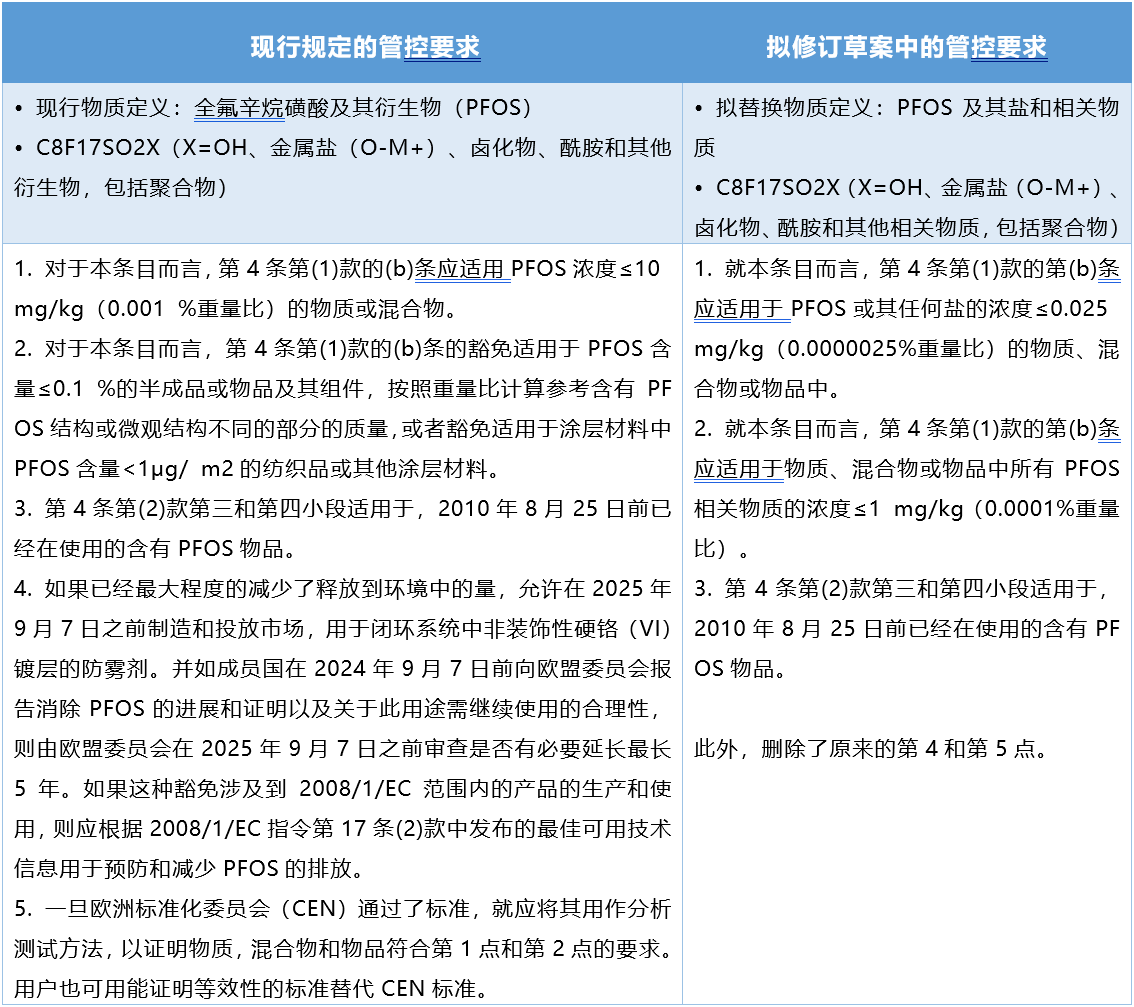
1. Redefines the definition of PFOS.
2. The concentration of PFOS and salt was adjusted from 10 mg/kg to 0.025 mg/kg.
3. The concentration of PFOS related substances was adjusted from 1000mg/kg to 1mg/kg.
4. The specific exemption of PFOS has been deleted, and it is considered that there is no need to set the exemption clause.
The comparison between the current requirements of PFOS and the proposed revised draft is as follows:
Warm tips
The EU POPs laws and regulations are updated frequently and the enforcement is constantly strengthened. Cases of related products being notified illegally due to violating POPs laws and regulations abound. ZRLK suggested that relevant enterprises should always pay attention to the dynamics of laws and regulations, formulate control measures for enterprises, and reduce the economic losses caused by products violating POPs laws and regulations. Our company has a wide range of testing fields and a professional and efficient service team, which can help enterprises evaluate the special chemicals regulated in products and make your products meet the corresponding national and international organization standards. If you need it, please feel free to contact us, and our engineers will serve you at the first time!













