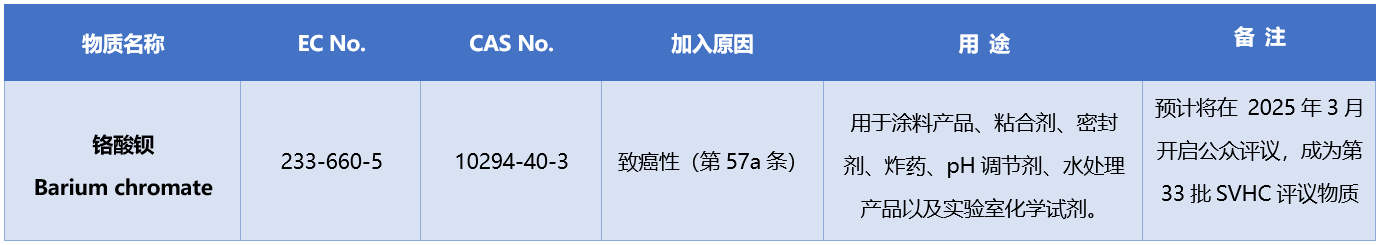
On August 6, 2024, the European Chemicals Agency (ECHA) added barium chromate to the SVHC substance of interest. ECHA plans to launch a public review of this substance in March 2025. If the review is approved, it will be officially added to the SVHC candidate list.
The specific information of the newly added intended substance is as follows:
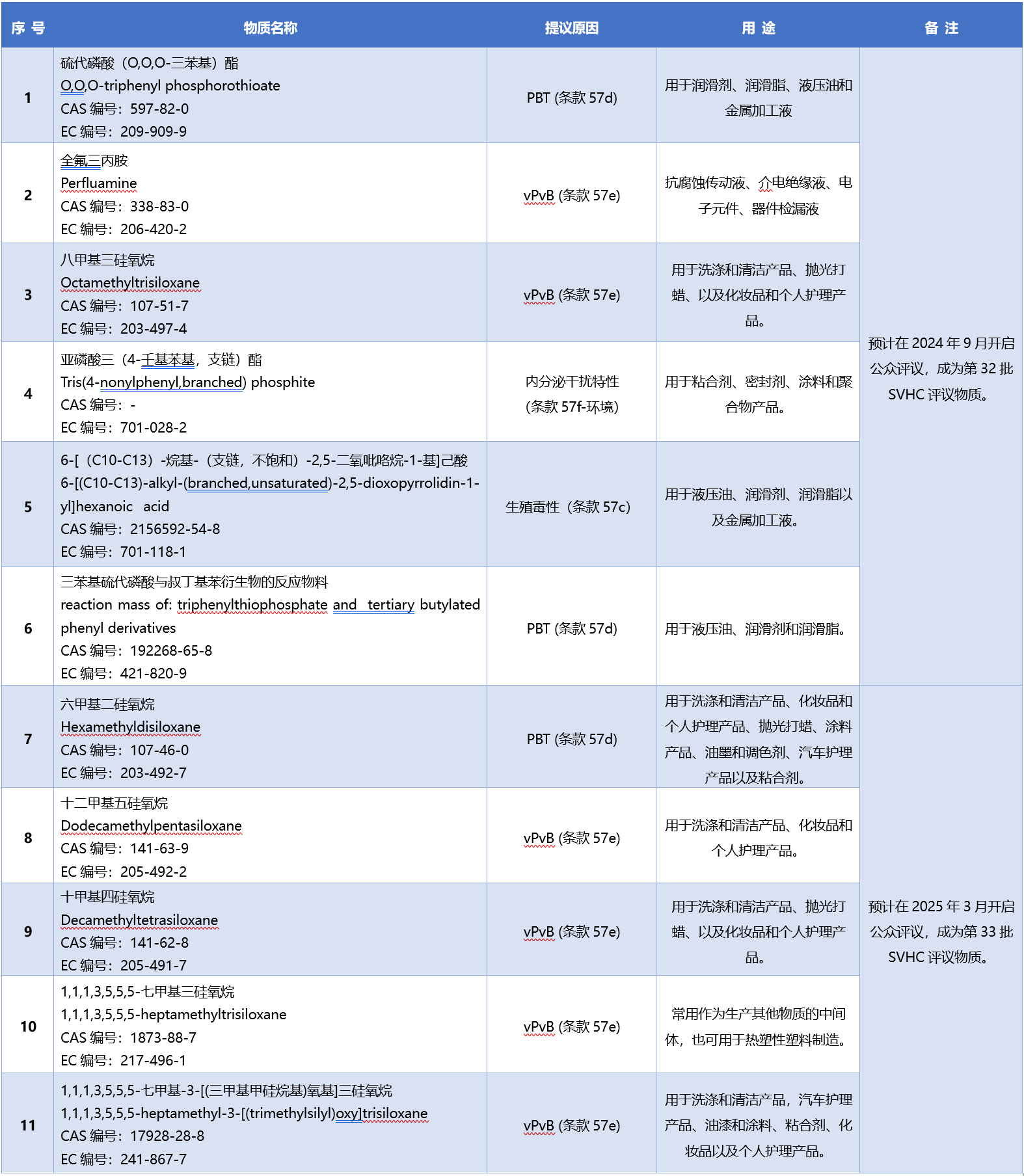
As of now, the number of REACH SVHC substances of interest has increased to 12, all of which are in the stage of "the proposer preparing the relevant substance files". The information on the other 11 intended substances is as follows:
Regulatory requirements for SVHC substances:
REACH regulation stipulates that when the SVHC content in an item exceeds 0.1%, information transmission must be carried out within the supply chain; When the SVHC content in an item is greater than 0.1% and the annual export volume is greater than 1 ton, it must be reported to ECHA.
According to the EU Waste Framework Directive, starting from January 5, 2021, suppliers who release items with SVHC content exceeding 0.1% into the EU market should submit relevant information about the items to ECHA through the SCIP database.
Kind reminder
With the continuous updating of the SVHC candidate list, enterprises are facing increasing control requirements. ZRLK suggests that relevant companies increase their risk awareness of their products, conduct early investigations into their supply chains, and constantly monitor updates to REACH regulations to calmly respond to regulatory changes. Our company has a professional technical team and rich experience in product testing, which can help you easily understand whether the product is safe and compliant. If you need it, please feel free to contact us at any time, and our engineers will be at your service as soon as possible!













