On December 16, 2020, the European Commission issued Regulation (EU) 2020/2096, which extensively revised a number of requirements in the EU REACH Annex XVII Restricted Substance List.
Regulation (EU) 2020/2096's amendments to REACH Annex XVII mainly include: deletion of some clauses in REACH Annex XVII that have stricter restrictions in the EU POPs Regulation (EU) 2019/1021; revision of some clauses to make them Restriction requirements are more clarified; some test methods for restricted substances are updated. The main amendments to REACH Annex XVII of Regulation (EU) 2020/2096 are as follows:
REACH Annex XVII related clauses | modify the content |
Item 3 | The relevant content in the restriction requirements in column 2 has been revised as follows: 1. Amend paragraphs 3 and 5, delete the content about the R65 label; 2. Delete paragraphs 6 and 7. |
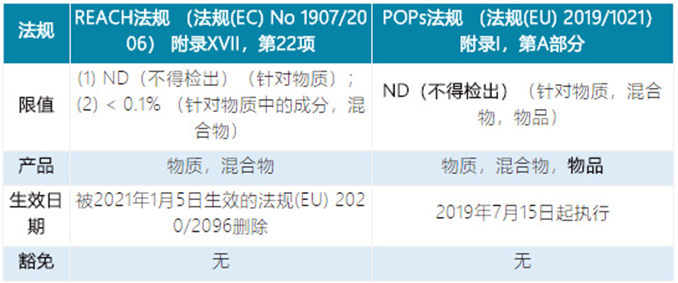
Item 22 | Delete the restriction requirements of pentachlorophenol (has been included in the POPs regulations) |
Items 28-30 | The second column restriction requires the following content to be added to the second paragraph: ‘(F) Equipment covered by Regulation (EU) 2017/745’ |
Item 46 | Delete the CAS number and EC number cited in paragraph (a) in column 1 |
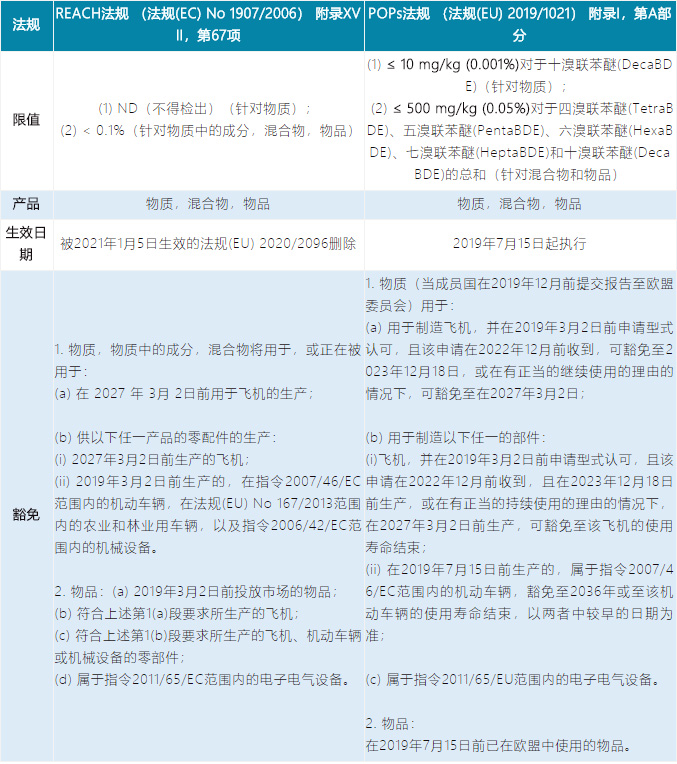
Item 67 | Delete the restriction requirement of Decabromodiphenyl ether (has been included in the POPs regulations) |
Item 68 | Delete the restriction requirements of PFOA, its salts, and PFOA-related substances (have been included in the POPs regulations) |
In addition, the regulations have revised the relevant content in Annex XVII of REACH: 1. Revise part of the information in Annex 1-6 to update the classification of CMR substances and add some new substances listed as CMR 1A and 1B; 2. Update the test method for azo dyes in the 43rd restriction clause in Annex 10. | |
Regulation (EU) 2020/2096 will come into effect 20 days after the official gazette is published. The amendment to delete the 68th item of PFOA, its salts, and PFOA-related substances restrictions has become effective on July 4, 2020. The effective date of some amendments in Annex XVII of REACH is also stipulated in the amendment regulations.
The similarities and differences of the requirements for substances 22, 67 and 68 in REACH and POPs regulations:
Item 22: Pentachlorophenol (PCP) and its salts and esters
Item 67: Decabromodiphenyl ether (DecaBDE)
Item 68: Perfluorooctanoic acid (PFOA) and its salts and PFOA related substances














