On May 3, 2021, the US Consumer Product Safety Commission (CPSC) announced the direct final regulations to amend 16 CFR Parts 1107 and 1112.
The new regulations will take effect on July 29, 2021.
The ISO/IEC 17025 and ISO/IEC 17011 versions are updated, and the relevant standards mentioned in 16 CFR Part 1107 and Part 1112 are revised as follows:
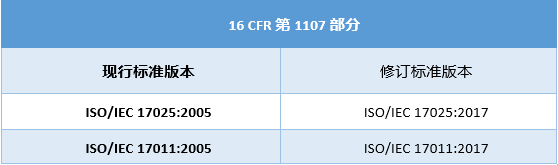

Note:
16 CFR Part 1107 related to product certification testing and labeling
16 CFR Part 1112 on the requirements of third-party conformity assessment agencies
ISO/IEC 17011 Conformity Assessment-Requirements for Certification Bodies of Conformity Assessment Bodies
ISO/IEC 17025 General requirements for the competence of testing and calibration laboratories













