Recently, the UK Health and Safety Commission (HSE) released the UK REACH 2021/2022 annual work plan, of which four items need to be paid attention to: transitional policy and formal registration, dossier completeness evaluation and test proposal evaluation, substance evaluation, SVHC list, authorization List and restriction list.
1. SVHC list/authorization list and restriction list
In 2021-2022, HSE, the UK Environment Agency and relevant authorities are considering including the following substances in the list of substances of very high concern (SVHC):
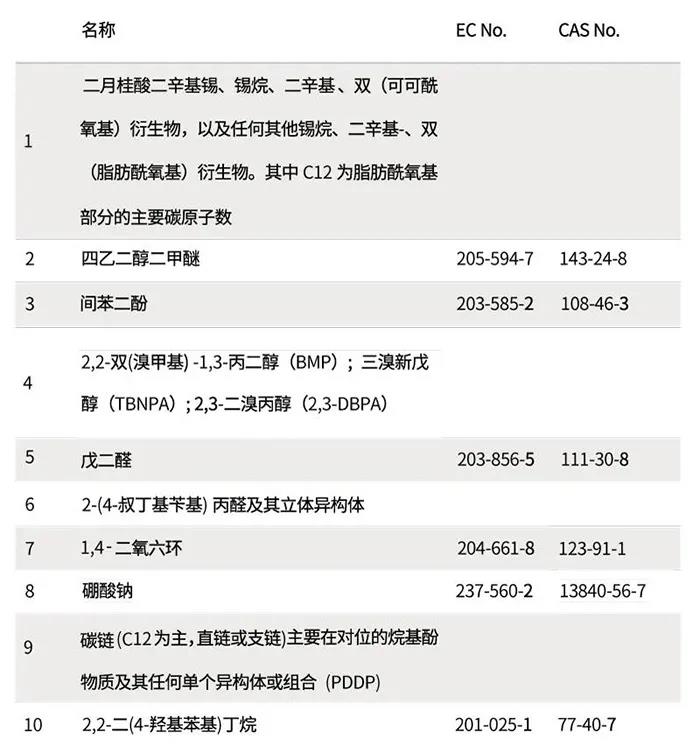
Note: HSE is considering including some of the substances in the SVHC list in the authorized list, and plans to conduct the first primary selection before the end of this year. Before the substances included in the authorization list are put on the market or used in the UK market, British manufacturers and producers need to submit an authorization application to HSE.
HSE will initiate restriction proposals for the following substances in 2021-2022:
◆ Lead ammunition. Widely used in the shooting industry, it will cause harm to the environment, wildlife and humans;
◆ Harmful substances in tattoo ink. May be harmful to human health, the most common is to cause skin irritation or sensitization. The assessment of these substances will include but not limited to their carcinogenicity, reproductive toxicity, skin irritation and sensitization.
2. Material evaluation
The RAP list is updated once a year, and the first RAP draft list will be published before March 31, 2022.
RAP is HSE's close cooperation with the British Environment Agency to carry out substance assessment, launching the "Rolling Action Plan" (RAP), and adding registered substances to the RAP list according to the hazard classification, exposure and tonnage of the substance.
3. Dossier completeness evaluation and test proposal evaluation
UK REACH is consistent with EU REACH. HSE will also evaluate the completeness of the registration dossier and the rationality of the test proposal, and issue a decision.
4. Transitional policy and formal registration
As of February 24 this year, HSE has received 2,607 registrations and 15,720 downstream user import notifications (DUIN).
The DUIN notification will end on October 27 this year. All companies that have registered with EU REACH and want to maintain access to the UK market are requested to act as soon as possible to ensure compliance of their products in the UK market. Official registration period of 2, 4 or 6 years from the start.













