On September 17th, 2025, the European Chemicals Agency (ECHA) withdrew "dodecyl pentasiloxane" from the list of intended substances of SVHC. At present, there are no substances listed in the SVHC intentional substance list.
The information of the revoked intentional substance is as follows
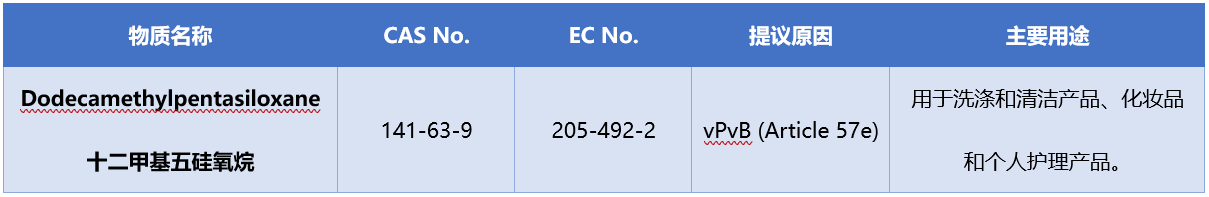
The related laws and regulations of SVHC substance require:
REACH regulations stipulate that when the content of SVHC in articles is greater than 0.1%, information must be transmitted in the supply chain; When the content of SVHC in articles is more than 0.1% and the annual export volume is more than 1 ton, it must be notified to ECHA.
According to the EU Waste Framework Directive, from January 5, 2021, suppliers who put goods with SVHC content exceeding 0.1% into the EU market should submit the relevant information of the goods to ECHA through SCIP database.
Warm tips
With the continuous updating of SVHC candidate list, enterprises are facing more and more control requirements. ZRLK suggested that relevant enterprises should improve their product risk awareness, investigate the supply chain as early as possible, and always pay attention to the update of REACH regulations, so as to calmly cope with the changes in regulations. Our company has a professional technical team and rich experience in product testing, which can help you easily understand whether the products are safe and compliant. If you need it, please feel free to contact us, and our engineers will serve you at the first time!













