On October 9, 2025, the European Chemicals Agency (ECHA) announced that the EU Member States Committee (MSC) has agreed to designate 1,1'-(1,2-ethylene)bis[pentabromobenzene] (decabromodiphenylethane, DBDPE) as a substance of very high concern, or SVHC, due to its very persistent and very bioaccumulative (vPvB) properties. ECHA plans to add the substance to the Candidate List in November, bringing the total number of substances on the official Candidate List to 251.
The following is relevant information about this substance
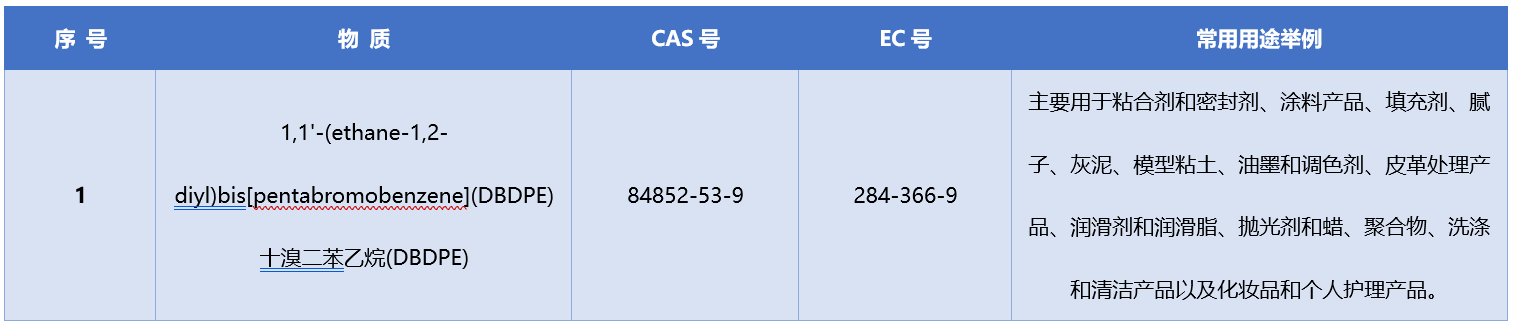
About Decabromodiphenyl Ethylene (DBDPE)
Decabromodiphenyl Ethylene (DBDPE) is a commonly used additive flame retardant. Due to its vPvB (Article 57e) properties, Sweden proposed DBDPE for inclusion on April 23, 2025. On June 2, 2025, the European Chemicals Agency (ECHA) launched a public consultation on the proposed inclusion of DBDPE on the SVHC list, with public comments closing on August 11, 2025. In October 2025, the Member States Committee (MSC) agreed to designate DBDPE as a substance of very high concern.
Warm Tips
The SVHC list is updated twice a year. With the continuous updates to the SVHC Candidate List, companies face increasing regulatory requirements. ZRLK recommends that relevant companies enhance their product risk awareness, conduct early supply chain investigations, and monitor REACH regulatory updates to ensure they are prepared to respond to regulatory changes. Our company has a professional technical team and rich experience in product testing, which can help you easily understand whether the product is safe and compliant. If you need it, please feel free to contact us and our engineers will serve you as soon as possible!













